Introduction
Thrombotic Microangiopathy (TMA) is a rare, life-threatening severe condition characterized by microangiopathic hemolytic anemia, thrombocytopenia and microthrombi in the walls of small blood vessels leading to ischemic organ dysfunction. Transplant-associated TMA (TA-TMA) can occur as a complication of allogeneic hematopoietic stem cell transplantation (allo-HSCT). The mechanism of TA-TMA is not fully understood, and conventional treatment options are based on targeting the identified risk factors and hypothesized pathophysiology. This review aimed to compile the information to date about TA-TMA and possible treatment strategies and report successful cases at the National Center for Cancer Care and Research (NCCCR).
Methods
Scientific databases were scoped for reviews on TA-TMA, its pathophysiology, risk factors and therapeutic strategies. Results were compiled to formulate an understanding of TA-TMA. Local cases of TA-TMA at NCCCR were also reported.
Results
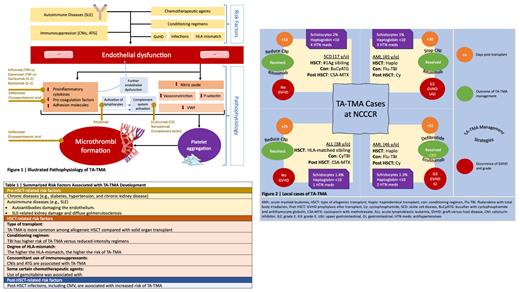
TA-TMA pathophysiology and risk factors are described in Figure 1 and Table 1, respectively. Due to the complexity of the patient cases under investigation, TA-TMA diagnosis is clinical. Proposed diagnostic criteria include anemia, thrombocytopenia, elevated LDH, presence of schistocytes, hypertension, proteinuria, and elevated sC5b-9. Therapeutic approaches start the management of modifiable risk factors such as withdrawal or decreasing the dosage of calcineurin inhibitors (CNIs), control of hypertension and AKI, treatment of active infections and management of graft versus host disease (GvHD). Therapeutic plasma exchange (TPE) may benefit some patients who are positive for factor H autoantibodies. eculizumab, a humanized antibody against complement protein, can be highly effective in patients with TA-TMA. It is recommended for patients with high-risk features such as elevated sC5b-9 and proteinuria and can also be used for patients with organ damage or disseminated disease. Other available treatment options include rituximab and defibrotide. Other therapeutic agents are under clinical trials such as ravulizumab (C5 inhibitor), nomacopan (C5 and leukotriene B4 inhibitor) and narsoplimab (mannan-binding lectin-associated serine protease-2 [MASP-2] inhibitor). Four cases were diagnosed with TA-TMA at NCCCR between September 2017 and February 2023, as described in Figure 2. All cases recovered; however, the challenge remains in the choice of therapy. Thus far, the choice is to individualize therapy according to comorbidities, severity of clinical presentation and availability of the treatment options.
Conclusion
TA-TMA remains a significant clinical challenge for transplant physicians, and more research is needed to improve our understanding of its pathogenesis, diagnosis, and management, particularly in guiding the choice of therapy.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal